Tissue Engineering and Regenerative Medicine in a Nutshell
Anushka Kore
Aspiring Biomedical Engineer
Thadomal Shahani Engineering College
Throughout the history of humankind, the prospect of artificially generating a tissue, organ or a complex living organism from a few novel cells has been deemed implausible by many. However, advances in clinical medicine from the last few decades have made this very vision feasible and one of the most sought-after avenues in terms of research. Tissue engineering and regenerative medicine (or simply, TERM) are the fields of biomedicine that deal with the implementation of these fundamental ideas to practical approaches. Hence, it is important to have a basic understanding of the principles of TERM to gauge its impact on clinical medicine in the future.
What is TERM, and why is it important?
When tissues or organs are so severely damaged due to traumatic injury, disease or congenital anomaly that conventional pharmaceutical treatments are no more applicable, the first step taken into consideration for the reconstruction of the diseased specimen is organ (or tissue) transplantation. But these surgical interventions are not without drawbacks. Current challenges faced by organ transplantation include a shortage of donated organs and immune rejection, despite advances in immunosuppressive therapy.
The scientific world was thus encouraged to think about an alternative approach which could eliminate the prospect of tissue and organ transplantation in the future. Although one such potential technique pre-existed, it gained real impetus when that technique was eventually coined as ‘tissue engineering’ by the scientific community.
In 1993, Langer and Vacanti defined tissue engineering (TE) as “an interdisciplinary field that applies the principles of engineering and life sciences towards the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ” (Langer & Vacanti, 1993). On the other hand, regenerative medicine (RM) has been defined as “the process of replacing or regenerating human cells, tissues or organs to restore or establish normal function” (Mason & Dunnill, 2008).
Simply put, both TE and RM deal with the restoration, maintenance and improvement in functions of the damaged tissues or organs. The difference, however, lies in the method used for attaining the objective. While the development of tissue in TE is in vitro, RM focuses on treating the damaged specimen by harnessing the body’s own regenerative capabilities (using stem cells or progenitor cells) which allows it to repair and restore itself to normal functions.
Due to their similar objectives, these two fields have been often used synonymously with each other, merging in recent years and originating in the broad field of tissue engineering and regenerative medicine (TERM).
How does it work?
To understand how TERM works, we need to dive into the fundamental constituent of the human body- the cell. Cells, as we know, are the structural and functional entities of a living organism. Tissues are formed when groups of cells similar in structure weave together to perform a specific function. Normally, such group of cells make and secrete their own support structures, known as the extra-cellular matrix. This matrix is more than just supportive in nature; it also acts as a relay station for various signaling molecules. Thus, a cell receives signals from various sources in its local environment and each signal can elicit a chain of reactions that determine the pathway taken and, ultimately, the fate of the cell. By understanding how individual cells respond to signals, interact with their environment, and organize into tissues and organisms, researchers have been able to manipulate these processes to mend damaged tissues or even create new ones.
To replicate this process in vitro, we require three ingredients- cells (mostly stem cells), that can differentiate and replace the damaged cells or tissue; scaffold or artificial extra-cellular matrix (ideally porous and biodegradable), which can hold and support the differentiating cells to mould them into the desired structure; and growth factors to encourage the cells to grow and develop into a tissue. Together, these three elements form the ‘pillars’ of TE.
The process can be initiated by introducing these elements one-by-one or in an alternative approach, the cells, scaffold and growth factors are mixed all together at once, allowing the tissue to ‘self-assemble’. Both these techniques are termed as in vitro TE (or, ex vivo TE) as the cell-scaffold construct is monitored and allowed to grow outside the body and then implanted to the specific site. Thus the in vitro technique of TE allows for the production of multiple engineered tissues from a single cell source.
Yet another approach involves enmeshing the scaffold with growth factors and then introducing stem cells from the patient into the framework. This construct is then implanted in the patient’s body until a new tissue is regenerated in vivo. Hence, this approach is appropriately termed as in vivo TE. This method of using the patient’s own cells to make customized tissues is advantageous as it reduces the chances of the immune system rejecting the newly generated tissue. However, in vivo TE serves only a certain single patient at a time, eliminating the prospect of mass-production of engineered tissues. Yes, a single stem cell source can be used to create multiple tissues, but this would increase the infection risk and the chances of rejecting the tissue by the immune system of patients other than the one from whom the stem cells were sourced. When a patient’s own cells are used, there is no infection but contamination risk alone.

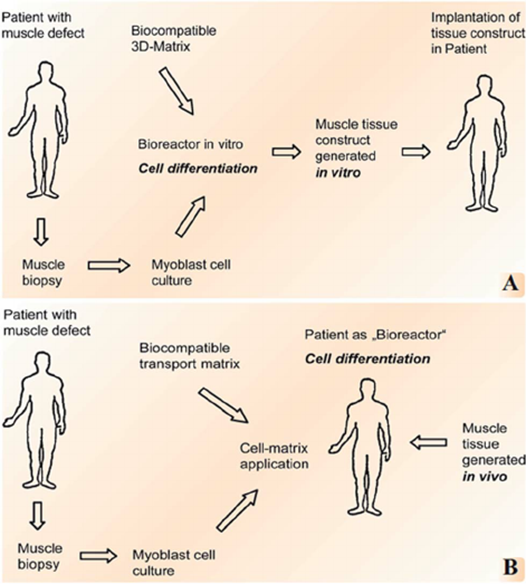
Depending on the patient’s diagnosis, the approach used might be different. Nonetheless, both tactics of TERM regenerate the patient’s tissue or organ that are entirely free of poor biocompatibility, low bio-functionality and severe immune rejection. This opens up many new opportunities in clinical medicine that might have not been possible with conventional medical practices.
The repair and regeneration of cartilage, such as in ears and joints, has been made possible by TERM which not only matches the appearance but also the function of native tissue (Watson, 2009). Similarly, skin substitutes prepared by TE (commonly known as ‘artificial skin’) could be used to promote acute and chronic skin wound healing (Tang et al., 2017).
Bone defects such as deformed structure due to traumatic injury or congenital conditions can be resolved by developing ideal bone substitutes through the principles of TERM. For bones with irregular shapes, 3D super-elastic scaffolds have been recently developed based on electro-spun SiO2 nanofibers with self-fitting and tailored gradient capability (Wang L.H. et al., 2019).
Likewise, development of corneal repair and regeneration (Han et al., 2020), treatment of tendon/ligament injury (Chen J. L. et al., 2010), cardiovascular TE pertaining to the construction of arteries, heart valves and myocardium substitutes (Yang et al., 2016) and regeneration of dental tissues (Han et al., 2020) has been made possible due to the advent of TERM.
Challenges and Future prospects
The applications of TERM hold the promise of custom-made medical solutions for injured and diseased patients. That, coupled with the development of new technologies focussing on material and scaffold generation, can be a game-changer, significantly impacting the future of TERM.
Techniques like microfabrication and micro-electromechanical systems (MEMS) can provide features approaching the size scale and complexity of the tissue generation in vivo. Two challenges, however, that could be encountered may be- problems associated with ‘scale-up’ and cell death associated with implantation. Large numbers of cells are required to generate relatively small volumes of tissue. To ultimately be effective in humans, it will be necessary to generate relatively large volumes, starting with very few cells.
Mature cells, expanded in vitro, lose efficacy. To combat this, different cell types that can be expanded in vitro and could also survive a relatively hostile environment at the time of implantation are being explored.
To be effective, the cells used should be easily procured, effectively expanded in vitro, survive the initial implantation, be accepted as self; and not recognized as foreign, and function normally and not become malignant. In addition, it would also be quite convenient if no moral concerns or questions were generated as a result of the cell type used.
Any type of research or scientific development is forever an ongoing process and there’s no doubt that new techniques to tackle the pre-existing limitations are being explored even as I write this article. As TERM cements itself as one of the central disciplines in biomedicine today, it will be interesting to see where the field will head and how it would transform clinical medicine as we perceive at the moment.
Reference (Feb-21-A6)

