Monoclonal Antibodies and their Applications for Treatment of COVID-19
Neil Sheth
BE Biotechnology
Thadomal Shahani Engineering College
Antibodies are proteins that are produced by the human immune system in response to invasion by a foreign substance, called an antigen. Antibodies work by binding to antigens and stimulate a series of chemical interactions, which lead to formation of an Antibody-Antigen complex, which our immune system then destroys. This is the fundamental concept behind the body’s ability to fight off and recover from infections.
What are Monoclonal Antibodies? (mAbs)
Monoclonal Antibodies are essentially the same natural antibodies, but produced artificially in laboratories and factories. They are not pharmaceutical products and contain no chemicals. They can bind only to the antigen they have specificity towards and are tailor made for various diseases. These compounds have been in use for over 30 years and have benefitted millions of people who could be immune compromised. Diseases such as rheumatoid arthritis, multiple sclerosis, SLE and various cancers have become manageable with the advent of mAbs. They are produced by cloning B-cells, and recognise single unique epitopes on an antigen which differentiates them from polyclonal Antibodies. Monoclonal antibodies are designed to be administered by injection. They are supplied as lyophilised powders or solutions for injection.
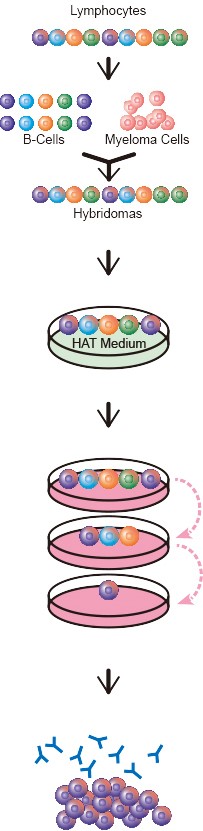
How are mAbs produced?
The first step is the collection of B lymphocytes from antibody producing organs such as spleen and lymph nodes. B lymphocytes have limited survival time and must now be immortalised by fusion with myeloma B cells using polyethylene glycol.
B-Cell and myeloma fusion is not 100% efficient. Therefore, selection for myeloma-lymphocyte hybrids is required. Hypoxanthine-aminopterin-thymidine medium (HAT) inhibits DNA synthesis via aminopterin. B-Cells and fused hybrids can overcome culturing in HAT medium, as they posses thymidine kinase which allows them to synthesize requisite DNA polymerase precursors from the HAT medium supplied thymidine. Myelomas do not produce thymidine kinase, and consequently do not survive in HAT medium. Although mortal B-Cells contain thymidine kinase, they eventually die off due to limited in vitro replication ability. The cells which survive selection are still heterogeneous, containing both multiple clones specific to the target antigen and clones producing antibodies with irrelevant specificity. Single cells are essential to assure clonality and achieved through limiting dilutions.
After these steps, the selected hybridomas are expanded and cryopreserved in stocks for safeguarding and preservation. This ensures batch consistency and reliable performance. In-vitro techniques such as roller bottle production allow for large scale production for mass distribution
mAbs for COVID-19
Encouraging results for Covid-19 antibody treatment have emerged from preclinical research and from initial clinical trials. An effective treatment regimen will largely benefit by the approval of mAbs for SARS-COV-2 since these therapies do not have any major side effects that a vaccine could possibly induce, and more importantly have the ability to cure rather than prevent. Large scale vaccinations are a challenging task to accomplish and could lead to various undesirable effects which are still unknown. More importantly, vaccines can only be administered to healthy individuals. Here mAbs can play a complementary role to vaccines and reduce the logistical burden of vaccinating a billion people.
Many experts believe COVID-19 will become an endemic or a permanently circulating disease, which makes it important to know just for how long the vaccine can protect an individual and whether he will have to take a booster dose once antibody levels subside. Moreover, we already know of the virus’ ability to mutate, and this could very well render the vaccine ineffective and require further research. With mAbs, the overall development time can be reduced and outpace the research and trial time a vaccine requires.
The most recent development on this front is an agreement between IAVI, Serum Institute of India and Merck KGaA to develop SARS-CoV-2 neutralizing monoclonal antibodies to address the pandemic and we should expect good progress soon.
Reference (Nov-20-A4)

